Scope of the project
Processes in a biogas plant
In a Biogas Unit, biogas (CH4 + CO2) is formed from the anaerobic digestion of waste. Anaerobic digestion is considered the most suitable route for the treatment of agro-industrial waste as, in addition to reducing the pollutant load of the waste and recovering energy, a digested residue is produced that can be used as a soil fertilizer. Over the last years, in Greece, significant investments have been made in the biogas sector. The trend of installing new units is predicted to be upward in the coming years. The feed of the units includes animal by-products such as food waste, whey, expired products, etc. (category 3 materials) and/or animal by-products and waste that were never intended for human consumption (livestock manure, slaughterhouse waste, etc.) (category 2 materials). If manure and/or category 3 materials are used, the biogas plants must comply with European regulations EU (no) 1069/2009 and EU (no) 142/2011 for safe handling of animal by-products.
The regulations are mainly concerned with eliminating the risk of spreading pathogens associated with the transport and disposal of the digestate. To ensure effective digestate sanitation, a controlled methodology must be applied to the treatment process. The Animal By-Products Regulation has established standard processing parameters that include a pasteurization unit in which the material must be subjected to a minimum guaranteed residence time (MRT) of 1 hour at 70°C with a particle size ≤12mm. The implementation of such a sanitation unit is often undesirable, as it has high investment and energy costs, high maintenance and use costs, thermal losses and generally leads to a reduction in the overall availability of the installation due to an increased risk of functional failure. In addition, spore-forming bacteria (e.g., Bacillus spp. and Clostridium spp.) survive pasteurization and anaerobic digestion, posing potential contamination risks to humans and animals. Another method suggested for the effective sanitization of most polluted feeds is sterilization at 133°C for 20 minutes, which is also unprofitable.
What is photocatalysis?
Thus, the need to develop and integrate into the biogas process, methods that are effective in eliminating the risk, repeatable and friendly to the environment and to the economic viability of biogas plants, is evident. Within this context, there is a growing interest in the use of Advanced Oxidation Processes (AOP: Advanced Oxidation Processes). One of the Advanced Oxidation Processes that has been extensively studied over the last decades is photocatalysis.
Photocatalysis is the acceleration of a photoreaction in the presence of a catalyst, which, without being consumed, reduces the activation energy of the reaction by offering an alternative mechanism for its realization. Photocatalysis is divided into two types, the homogeneous where the catalyst is in the same (liquid) phase as the suspension and the heterogeneous which includes two different phases.
Photocatalysts as potential disinfection agents
The use of photocatalysts as potential disinfection agents has been studied since the early 1990s. Matsunaga et al. were among the pioneers who exploited the use of the photocatalyst, titanium dioxide, to inactivate bacteria. Similarly, other conventional and unconventional catalysts were evaluated for their antimicrobial efficacy. Photocatalysis has been widely used to address many environmental challenges posed to modern society. The use of light to induce degradation as well as complete demineralization is a profitable green alternative.
Despite the remarkable technological advances seen in the last decade, there are still challenges and limitations that need to be addressed. Existing research data do not meet the requirements for high-throughput processes. However, many studies detailing structural and chemical modifications of photocatalysts to achieve an optimal energy gap for sunlight utilization and studies to search for new low energy gap and low frequency photocatalysts are in progress.
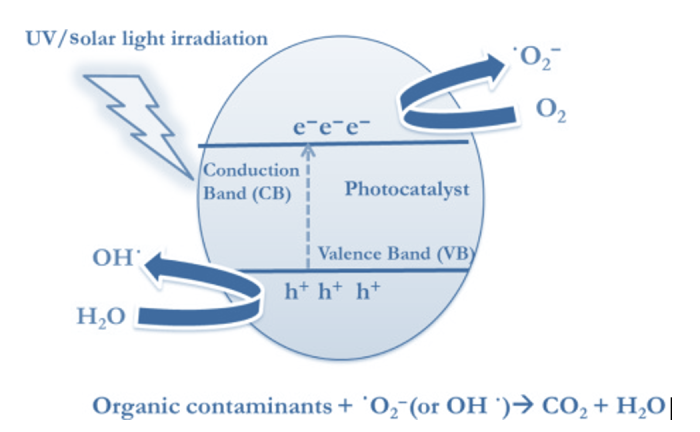
In the case of titanium, which is widely used to induce a series of redox and oxidation reactions on its surface due to the single electron of its outer orbital, the energy gap is 3.2eV. When a photon energy (hv) equal to or greater than the gap energy reaches the catalyst surface, the single valence electron will be excited from the valence band to the conduction band. The figure shows the mechanism. In the last decade, new photocatalysts have been chemically and morphologically modified using various nanomaterials aiming to increase radical production and to induce activation using visible light (solar) radiation.
Objectives of photoSAN
- Evaluation of the substrates/feeds and the corresponding digestate physicochemically as well as microbiologically using new molecular approaches
- Investigating the integration of an optimal liquid and solid fraction separation system for the separation of digestate
- Design of new photocatalytic materials to optimize photocatalytic disinfection
- Development of photocatalytic membranes using the materials developed
- Design and development of a sanitation reactor using the photocatalytic membranes in combination with the liquid and solid fraction separation system
- Pilot development of the technology and its integration in the anaerobic digestion plants

